
Agitation may cause partial coalescence of the fat globules (partial churning) which increases viscosity. Cooling temperature increase viscosity due to the increased voluminosity of casein micelle and temperatures above 65☌ increase viscosity due to the denaturation of whey proteinsĬooled raw milk and cream exhibit non-Newtonian behavior in which the viscosity is dependent on the shear rate. The viscosity of milk and dairy products depends up on the temperature and on the amount and state of dispersion of the solid components. V iscosity increases with increasing concentration of fat and solids-not -fat, but consistent general relationship could not be established This is due to the swelling of the micelles followed by their disintegration. Addition of alkali (pH up to 11.7), urea (up to 4.8 M) and calcium complexing agents to concentrated (22.7% solids) skim milk causes a marked transient increase of several folds in viscosity followed by a sharp decline. The viscosity is approximately doubled by the addition of 10 ml of 1.4 to 3.8 N ammonia to 90 ml milk. Changes in the caseinate micelles produced by either raising or lowering the pH results in increased viscosity. The viscosity of colloidal systems depends upon the volume occupied by the colloidal particles.

Important factors that influence the viscosity of milk are as follows:ġ0.2.1 State and concentration of the protein An increase or decrease in pH of milk also causes an increase in casein micelle voluminosity. The viscosity of these products depends on the temperature and pH. Milk and skim milk, excepting cooled raw milk, exhibit Newtonian behavior in which the viscosity is independent of the rate of shear.
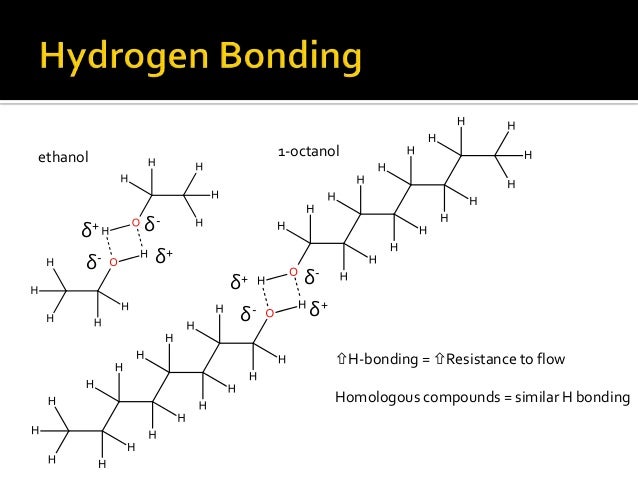
Viscosity of milk and milk products is important in determining the flowing rate of cream, rates of mass and heat transfer, the flow conditions in dairy processes. The viscosity of fluids is influenced by various factors and knowledge of which is of immense value in understanding the behavior of these fluids during processing.ġ0.2 Factors Influencing the Viscosity of Milk The viscosity of a heterogeneous substance such as milk at a given temperature depends upon its composition and the physica1 state of its colloidally dispersed substances, including milk fat. It is a measure of the friction between molecules as they slide past one another. Also, the contrary observation was found in the binary mixtures of acetone–ethanol and acetone–hexane, having a negative viscosity deviation.The viscosity of a substance refers to, its resistance of flow. The micelle surfaces are surrounded by hydration layers, leading to the positive viscosity deviation in the liquid mixtures because the water in hydration layers has a much higher viscosity than bulk water. This viscosity deviation can be mainly attributed to the formation of micelles of alcohol or acetone molecules in water because of the hydrophobic attraction between the hydrocarbon chains. It is shown that the mixing of water with the alcohols and acetone resulted in a positive deviation of viscosity, which reached the maximum value at the water mole fraction x 1 ∼ 0.7 for water–methanol, x 1 ∼ 0.72 for water–ethanol, x 1 ∼ 0.74 for water–propanol, and x 1 ∼ 0.83 for water–acetone binary mixture. "This articles studied and determined the viscosities of the binary mixtures of water–methanol, water–ethanol, water–propanol, water–acetone, acetone–ethanol, methanol–ethanol, and acetone–hexane and the ternary mixtures of water–methanol–ethanol and water–ethanol–acetone at 20☌. Viscosities of Binary and Ternary Mixtures of Water, Alcohol, Acetone, and Hexane
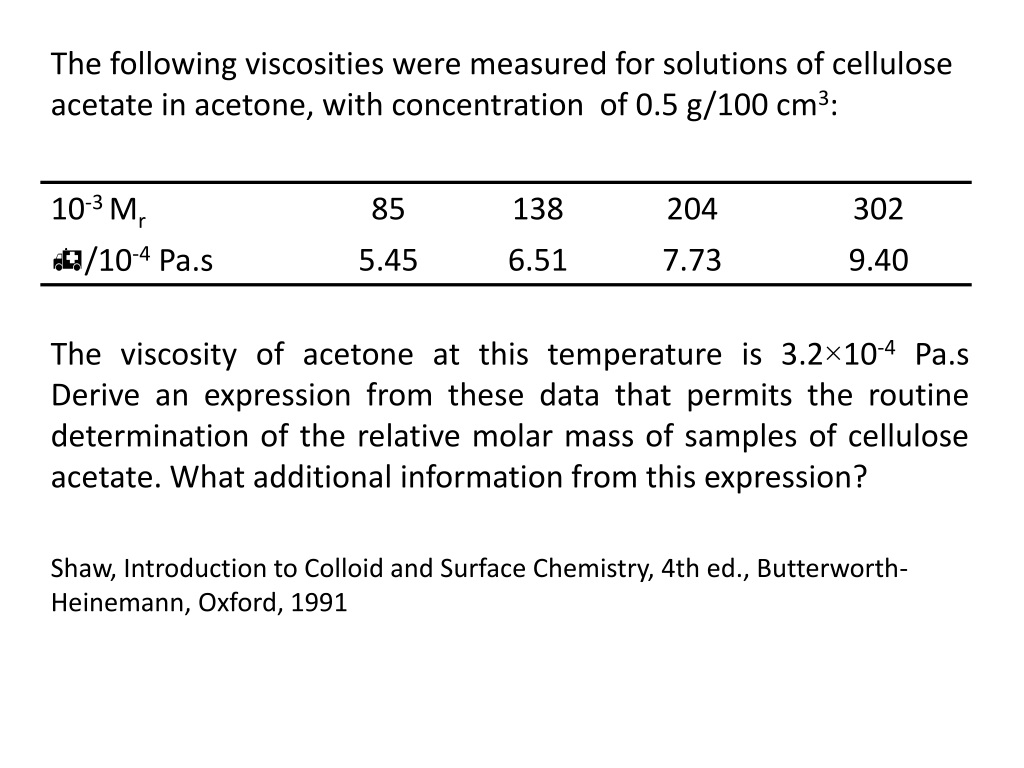
This answer just popped up when I searched for viscosity of water-ethanol mixtures, so I just copied the abstract:


 0 kommentar(er)
0 kommentar(er)
